
When iodine is heated, it produces vapour that can be collected in a test tube. To do this, the test tube should be inverted and placed over the source of heat. This allows the vapour to diffuse into the test tube.
What is the relation between diffusion and movements of particles in different states of matter?
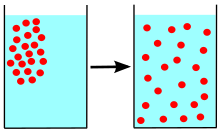
Diffusion is the process of particles moving from an area of high concentration to an area of low concentration. This process occurs in all states of matter, including solids, liquids, and gases. The rate of diffusion depends on the state of matter, with gases diffusing faster than liquids and solids. In solids, particles move around each other due to thermal energy, but diffusion only occurs when there is an imbalance of particles in an area. In liquids and gases, particles move freely and diffusion occurs more easily due to the lack of bonds between particles.
