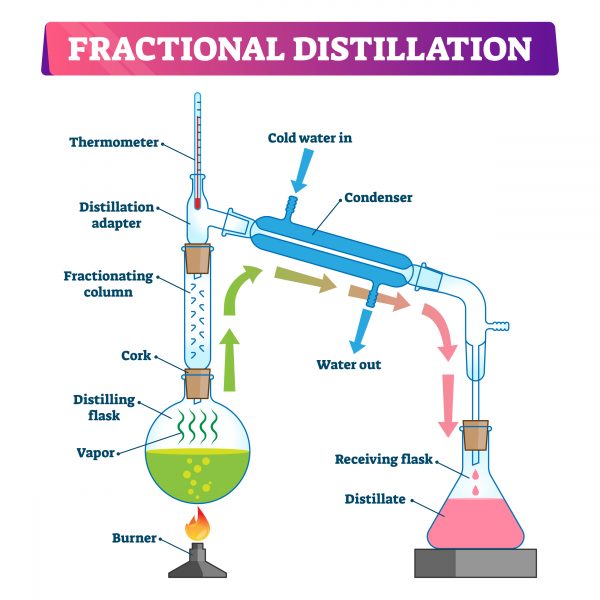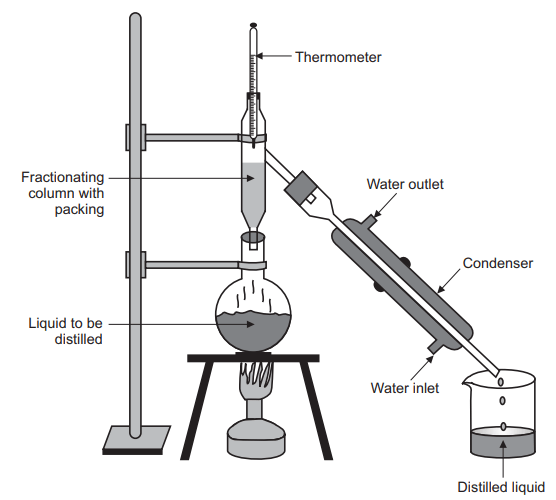
Fractional distillation is a method of separating a mixture of liquids into its components. It relies on the different boiling points of the components of the mixture. The mixture is heated until it reaches a temperature at which one of the components begins to vaporize, and the vapor is then collected and condensed back into a liquid. This process is repeated until all of the components are separated. Fractional distillation can also be used to separate liquids from solids, or to purify a liquid.
Fractional distillation is a process used to separate a mixture of liquids that have different boiling points. In this method, a round bottom flask is used as the distilling vessel. The flask is connected to a condenser and a thermometer. The mixture is heated and the vaporized liquid passes through the condenser, where it is cooled and condensed back into liquid form. The liquid is then collected in a receiving flask. As the vaporized liquid condenses, the components with the lower boiling points will condense first, while the components with higher boiling points will remain in the vapor phase. This process can be repeated multiple times, until the desired level of separation is achieved. For example, in the case of methanol and ethanol, the ethanol will condense out of the vapor phase first, followed by the methanol.