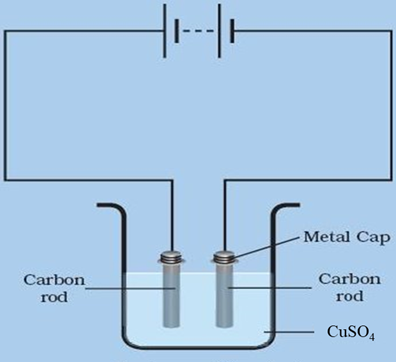
The carbon rod in the dilute copper sulphate solution with a cell potential of 1.2 V will act as the anode in the electrolytic cell. This means that the oxidation reaction of copper ions will occur at the carbon rod, and copper will be deposited on the surface of the rod.
Electrolysis
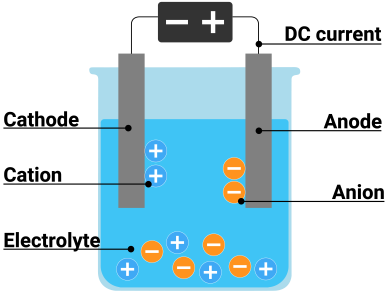
Electrolysis is a process in which electrical energy is used to break down substances into their component parts. It involves passing an electric current through a liquid or solution containing ions (atoms with an electrical charge). The electric current causes the ions to separate, forming separate elements. For example, when an electric current is passed through a solution of water, the water will break down into hydrogen and oxygen gas.
Electrochemical cell

Electrochemical cells are devices that convert chemical energy into electrical energy. They are made up of two electrodes (an anode and a cathode) that are placed in an electrolyte solution. When a voltage is applied to the electrodes, ions in the electrolyte solution move from one electrode to the other, creating
a current. This current can then be used to power electrical devices.
