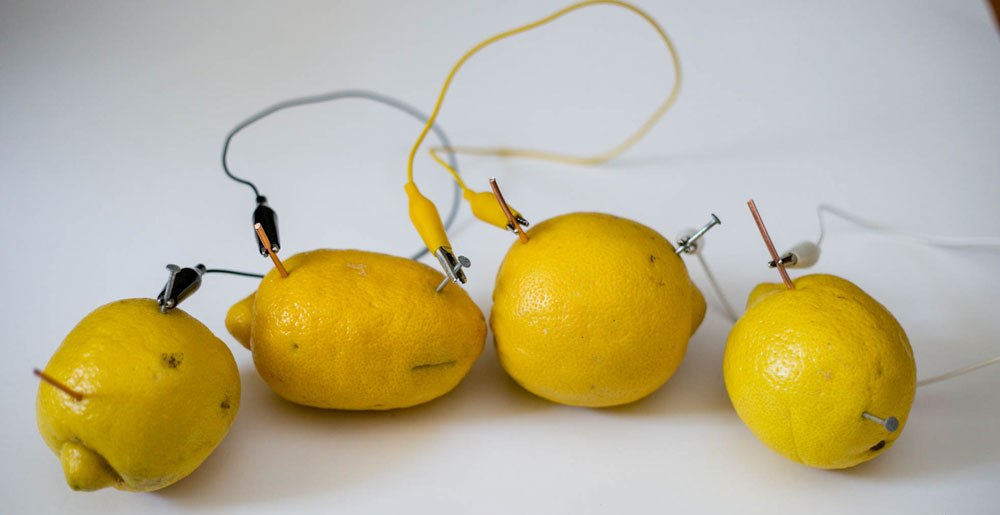
Lemon cells use the electrochemical reactions of two different metals (such as copper and zinc) immersed in a solution of lemon juice to create a current of electricity. The copper and zinc act as electrodes that attract and repel electrons, while the lemon juice acts as an electrolyte, allowing electrons to flow between the two metals. The reaction produces a small amount of electricity that can be used to power a small electronic device.
