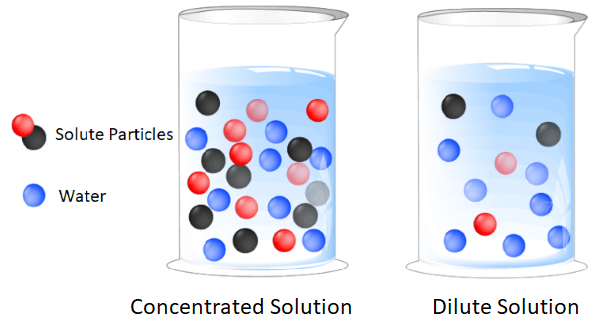
The concentration of a solution is a measure of how much of a solute is dissolved in a given amount of a solvent. It is usually expressed as a mass or volume fraction, or as moles of solute per liter of solution. The concentration of a solution can be changed by adding more solute or by adding more solvent. The concentration of a solution can also be changed by removing some of the solute or solvent. The concentration of a solution can be expressed in terms of molarity, molality, normality, parts per million (ppm), and parts per billion (ppb).
