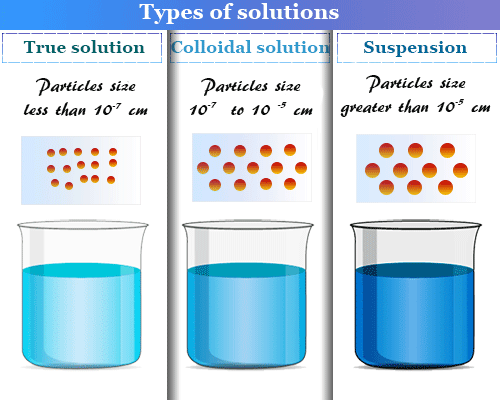
A colloid is a mixture of two substances, one of which is finely divided and dispersed evenly throughout the other. Examples of colloids include fog, whipped cream, and paint. A suspension is a mixture of two substances, one of which is larger and more dense than the other, and which settle out of the mixture over time. Examples of suspensions include mud, sand in water, and dust in air .The true solution is a homogeneous mixture of two or more substances, in which the particles of one substance are completely dissolved in the other. In a true solution, the particles of each substance are so small that they cannot be seen with the naked eye.
Experiment
- Copper sulphate + Water: When copper sulphate is added to water, it dissolves in the water and forms a clear, blue-green solution.
- Chalk Powder + Water: When chalk powder is added to water, it does not dissolve but instead forms a white, milky suspension.
- Milk + Water: When milk is added to water, it forms an opaque, white solution with a distinct odor.
Copper sulphate is soluble in water, while chalk powder and milk are not.
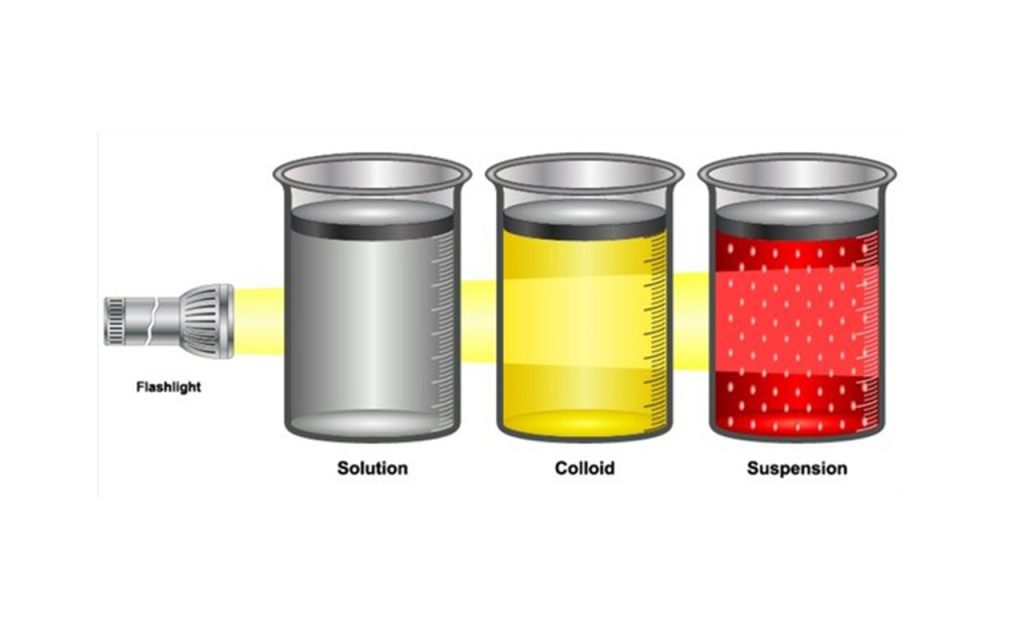
When you shine a beam of light on the three beakers, the copper sulphate and water solution will appear blue, the chalk powder and water solution will appear milky white, and the milk and water solution will appear cloudy white. The different colors are due to the fact that the copper sulphate solution is absorbing certain wavelengths of light that the other two solutions are not, giving it its distinct blue color.