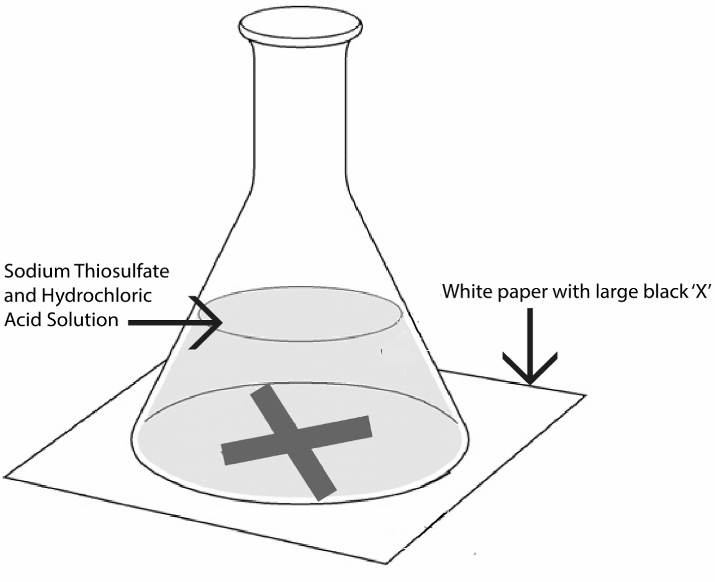
1. Place a beaker containing 50 mL of water on a flat surface.
2. Add 2g of Sodium thiosulphate to the beaker and stir the solution.
3. Place a light source (like a torch or a lamp) at a distance of about 15-20 cm from the beaker.
4. Arrange a path for the beam of light to pass through the beaker.
5. Add few drops of dilute HCl to the beaker and stir the solution.
6. Observe the solution for any change in the beam of light. You may notice the beam of light fading away as the reaction takes place. This is because the reaction between sodium thiosulphate and hydrochloric acid produces a precipitate of sulphur which scatters light and blocks the beam of light.
7. Stop stirring the solution and wait for the reaction to complete.
After stirring the solution, the beam of light will become visible and the experiment will be concluded. This is because the sodium thiosulphate reacts with the dilute hydrochloric acid to produce sulfur dioxide gas, which scatters the light.