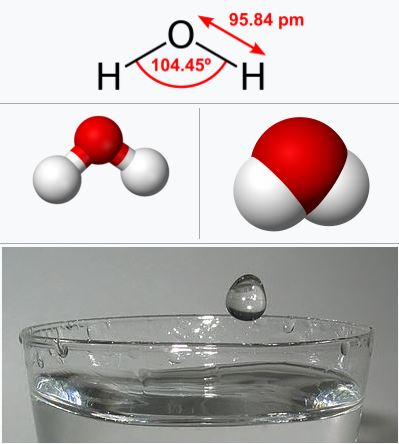
Water is a unique substance, because it is able to dissolve more substances than any other liquid. This is because of its polarity and the way its molecules interact with other substances. Water molecules are attracted to other polar molecules and can break apart the molecules, allowing them to be dissolved. This makes it a universal solvent, meaning it can dissolve a wide variety of substances, including salts, sugars, acids, bases, and many other compounds.
A neutral solvent is a solvent that has a neutral pH, meaning that it is neither acidic nor basic. Examples of neutral solvents include water, ethanol, propanol, and hexane.

