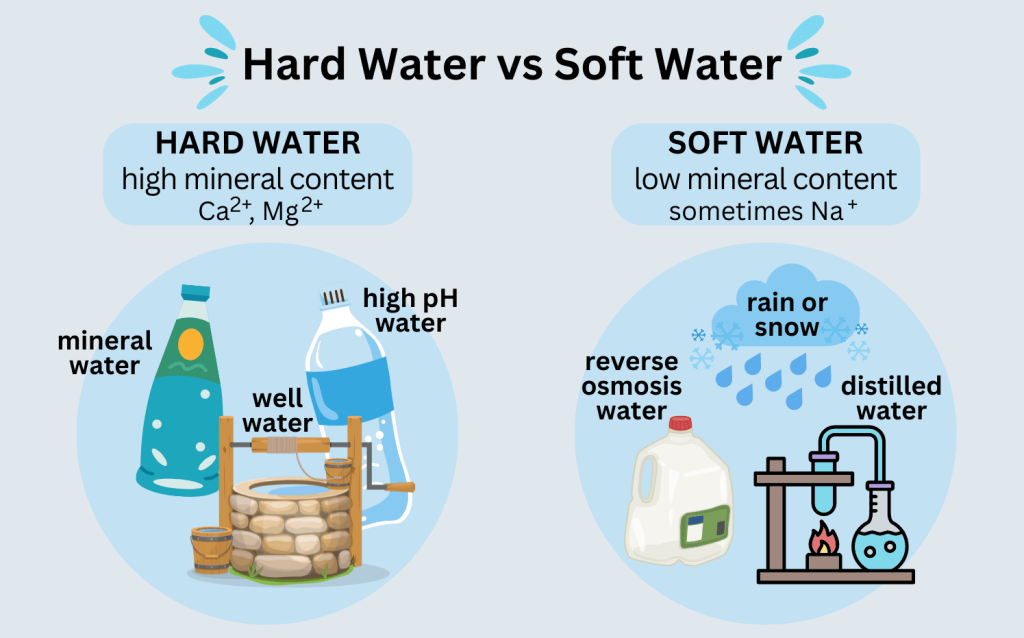
Soft water
Soft water is a type of water that has a low mineral content. It is composed of hydrogen and oxygen atoms, but does not contain significant amounts of calcium, magnesium, or other ions. Soft water is usually preferred for household use due to its low level of impurities, which makes it more suitable for bathing, laundry, and other household tasks. Additionally, soft water is often used in industrial settings, such as boiler systems, to reduce scale build up and improve efficiency.
Soft water can be obtained by using a water softener, which is a device that uses a chemical process to remove calcium and magnesium from the water. Softeners often use sodium or potassium ions to replace the calcium and magnesium ions in the water, resulting in a softer water that is more suitable for household use. Soft water can also be obtained by reverse osmosis, a process in which water is forced through a semi permeable membrane to remove impurities.
Overall, soft water is a type of water that is low in mineral content and is often preferred for household use due to its low level of impurities. Soft water can be obtained by using a water softener or reverse osmosis.
Hard water
Hard water is water that contains calcium and magnesium ions in significant amounts. These ions can impact the taste and feel of water, as well as its suitability for certain household uses. Hard water is generally not considered a health risk, but it can cause a variety of problems in the home, such as scale build up in pipes and on fixtures, spots on dishes and glasses, and soap scum on showers and tubs.
To treat hard water, a water softener can be installed. This device uses an ion exchange process to remove calcium and magnesium ions from the water, replacing them with sodium ions. The softened water is more suitable for household uses such as washing, bathing, and drinking.
Temporary hard water
Temporary hard water is water that is hard when it is first drawn from the tap, but becomes softer when heated. This is caused by the presence of calcium bicarbonate in the water, which breaks down when heated, leaving the water softer.
Permanent hard water
Permanent hard water is water that contains a large amount of dissolved minerals, such as calcium and magnesium, that cannot be removed by boiling or other methods. The minerals in permanent hard water can make it difficult to lather soap, cause build up in pipes and appliances, and leave deposits on fixtures. Permanent hard water can be softened with a water softener to make it easier to use and extend the life of plumbing fixtures.
