
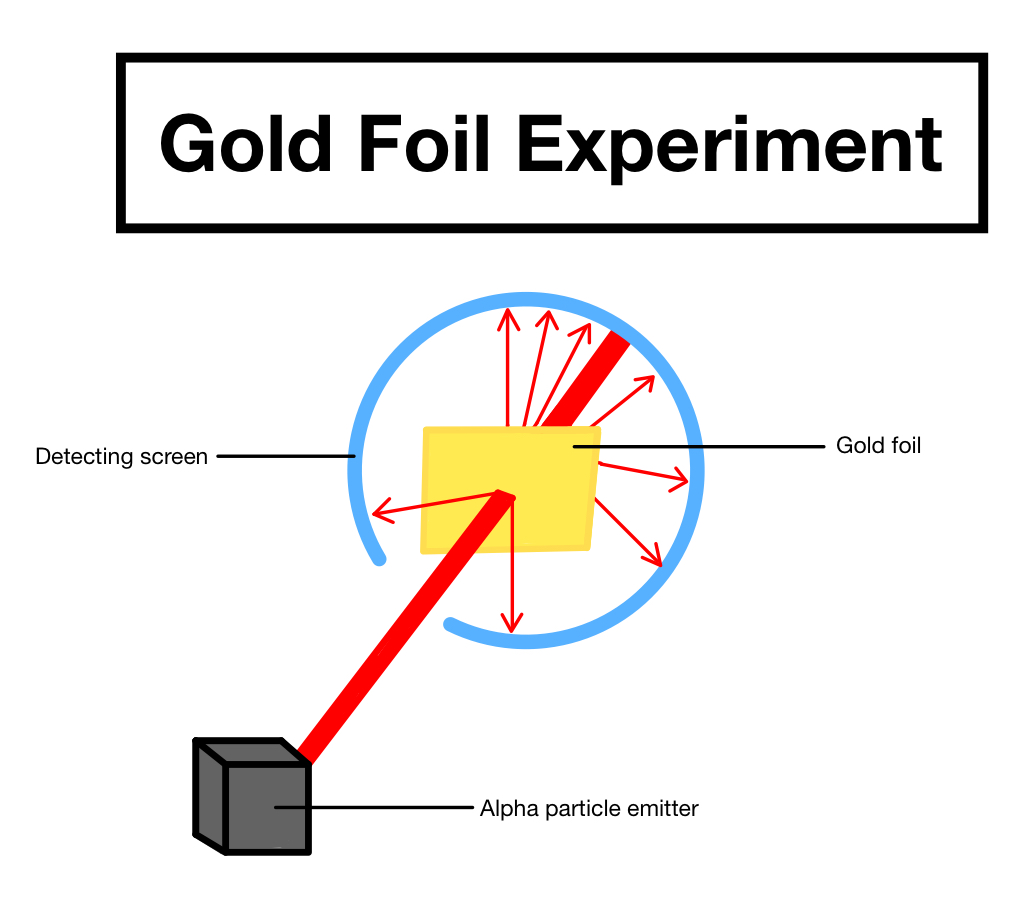
Ernest Rutherford is best known for his gold foil experiment, which was conducted in 1909. The experiment was designed to study the structure of the atom. Rutherford and his team bombarded a thin sheet of gold foil with alpha particles, a type of radiation. The team expected the particles to pass through the foil and continue in a straight line, as the atoms were thought to be mostly empty space. To their surprise, however, some of the particles were deflected, suggesting that the atom was not an empty void and had some kind of internal structure. This experiment provided the first evidence that the atom had a nucleus, and it revolutionized our understanding of atomic structure.
Ernest Rutherford’s famous “nucleus experiment” was conducted in 1911. In the experiment, Rutherford and his team bombarded a thin sheet of gold foil with alpha particles. He expected the particles to pass through the foil and continue in a straight line. Instead, some particles were deflected, while others were reflected back at the source. This provided evidence that the gold foil was made of particles much smaller than atoms, which Rutherford called nuclei. This experiment proved that the atom was not a solid and indivisible object, but instead composed of a nucleus surrounded by a cloud of electrons. It also provided evidence for the existence of the proton, which Rutherford identified as a positively charged nuclear particle.
enast, anast, rutherfod
