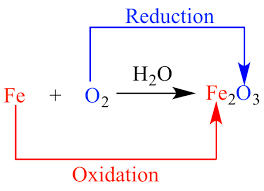
This reaction involves the transfer of electrons, with the oxygen atoms gaining electrons and the iron atoms losing electrons. The term “redox” is derived from the words “reduction” and “oxidation,” which refer to the gain and loss of electrons, respectively.
The oxidation of iron (Fe) to iron(III) oxide (Fe2O3) is an example of a redox reaction.
2Fe(s) + 3O2(g) → 2Fe2O3(s)
