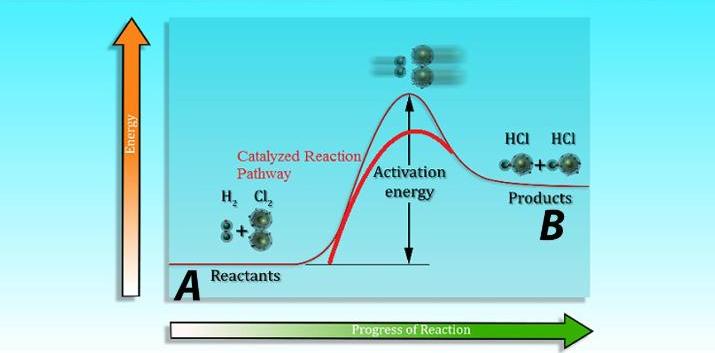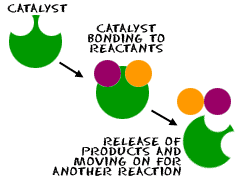
Catalysts are substances that speed up the rate of a chemical reaction without being consumed in the reaction. They do this by lowering the activation energy, the amount of energy needed for the reaction to begin. Different catalysts may be used depending on the reaction being catalyzed, and the rate of reaction will vary depending on the catalyst used. For example, the enzyme catalase is a catalyst used in the breakdown of hydrogen peroxide into water and oxygen, and its presence increases the rate of reaction by five orders of magnitude.