Skip to content
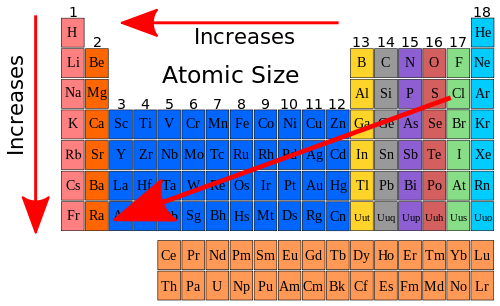
- Group 1: As the group number increases, the atomic radius of the element increases.
- Group 2: As the group number increases, the atomic radius of the element decreases.
- Group 3: As the group number increases, the atomic radius of the element increases.
- Group 4: As the group number increases, the atomic radius of the element decreases.
- Group 5: As the group number increases, the atomic radius of the element increases.
- Group 6: As the group number increases, the atomic radius of the element decreases.
- Group 7: As the group number increases, the atomic radius of the element decreases.
- Group 8: As the group number increases, the atomic radius of the element remains the same.