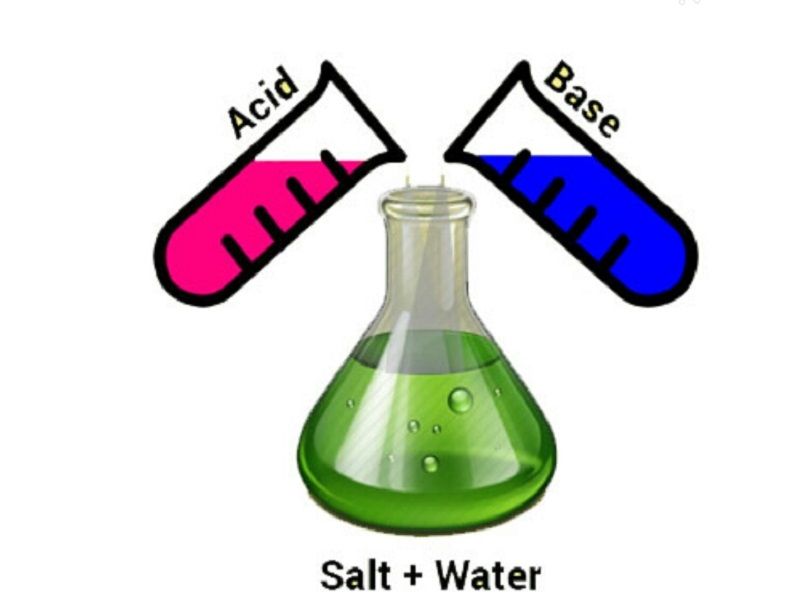
A neutralization reaction is a chemical reaction in which an acid and a base react to form a salt and water. The general chemical equation for a neutralization reaction is:
Acid + Base → Salt + Water
Examples of neutralization reactions include:
- HCl + NaOH → NaCl + H2O
- H2SO4 + 2NaOH → Na2SO4 + 2H2O
- HClO4 + KOH → KClO4 + H2O
- HNO3 + KOH → KNO3 + H2O
- H3PO4 + 3KOH → K3PO4 + 3H2O
