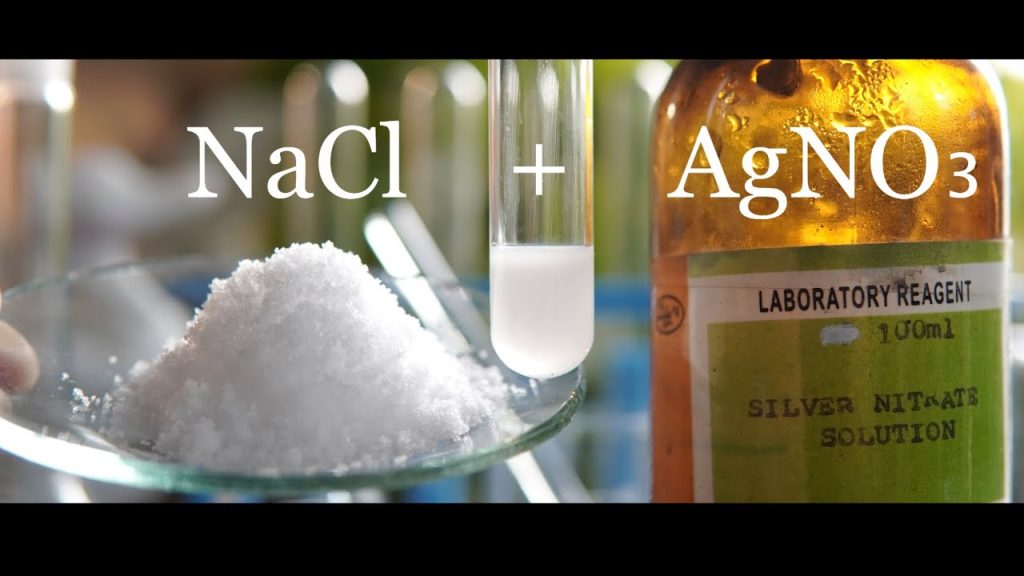
Chlorides are compounds that contain the chloride ion (Cl-). Common sources of chlorides include table salt (sodium chloride), bleach (calcium hypochlorite), and hydrochloric acid (HCl). Many other chemicals, such as potassium chloride, magnesium chloride, and ammonium chloride, are also chlorides.
Silver nitrate is a chemical compound with the chemical formula AgNO3. It is an odorless white solid that is highly soluble in water and has a relatively low melting point. It is used in photography, in the production of silver salts and other silver compounds, as a mordant in dyeing, and in medicine. When combined with a salt, such as sodium chloride, silver nitrate forms a precipitate known as a silver chloride disc. This disc is often used as a test for the presence of chloride ions in a solution.
The chemical formula for this reaction is AgNO3 + NaCl → AgCl + NaNO3.

