
To perform the experiment, you will need the following materials:
- A round-bottom flask
- A rubber stopper with a hole for a glass tube
- A glass tube
- A clamp stand
- A beaker
- A gas collection tube with a stopper
- A Bunsen burner
- Hydrochloric acid (HCl)
- Calcium carbonate (CaCO3)
Instructions:
- Set up the apparatus as shown in the diagram. Place the round-bottom flask on the clamp stand. Insert the glass tube through the rubber stopper and clamp it securely. Fill the beaker with water. Place the gas collection tube in the water so that the end of the tube is submerged. Secure the gas collection tube with the stopper.
- Measure out 10mL of hydrochloric acid and 10g of calcium carbonate into the round-bottom flask.
- Heat the round-bottom flask with the Bunsen burner. Aim the flame at the side of the flask and not directly at the bottom.
- Heat the flask until a steady stream of bubbles is released. This indicates that carbon dioxide
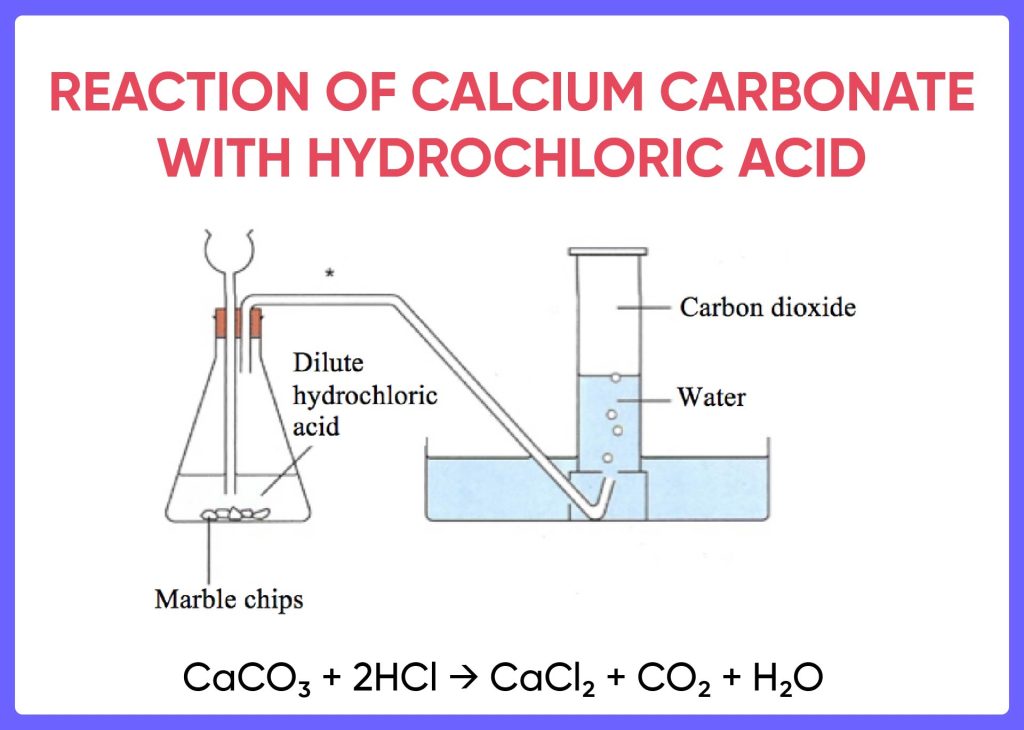
This experiment involves a reaction between hydrochloric acid (HCl) and calcium carbonate (CaCO3) to produce carbon dioxide (CO2).
Equation:
CaCO3 + 2HCl → CaCl2 + H2O + CO2
