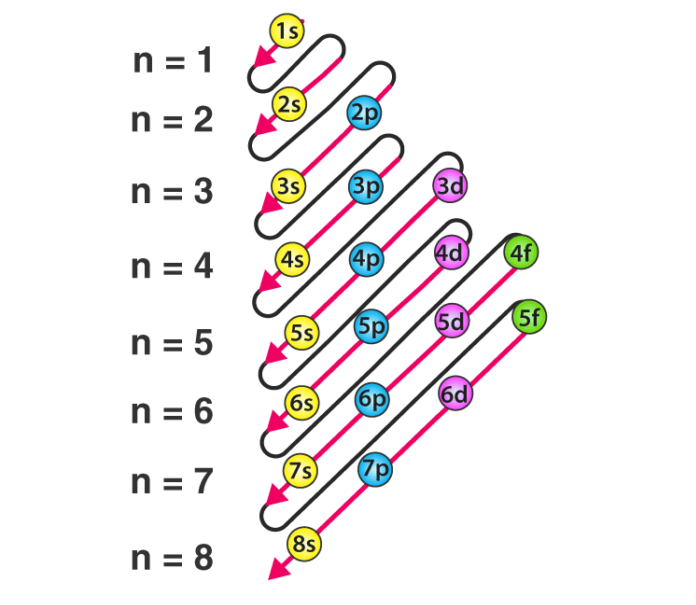
When filling electrons in a subshell, electrons are filled in order of increasing energy, beginning with the lowest energy level. The subshells are filled in the order of 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p.

When filling electrons in a subshell, electrons are filled in order of increasing energy, beginning with the lowest energy level. The subshells are filled in the order of 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p.