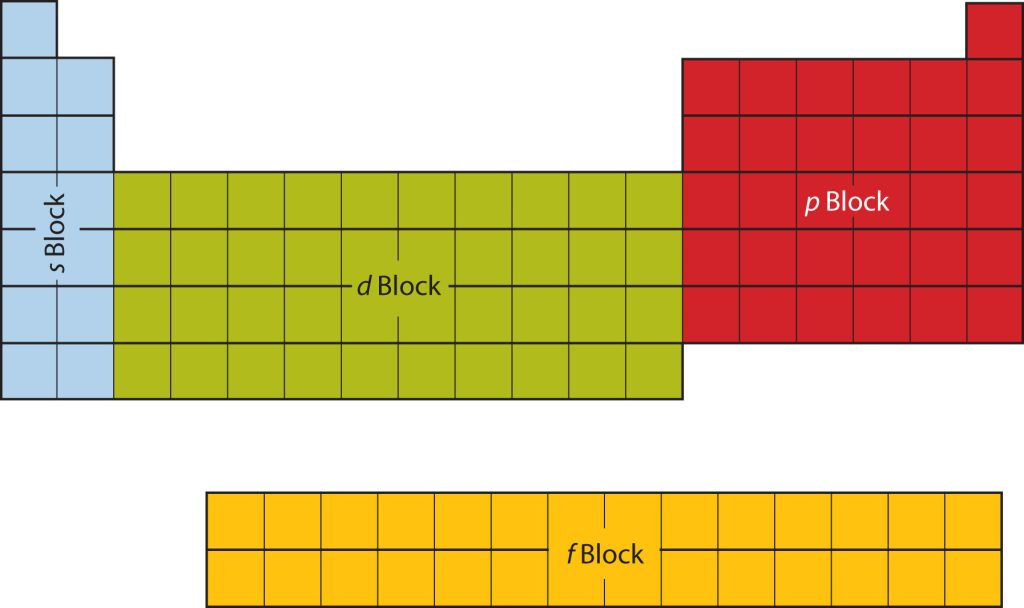
The subshell electronic configuration is the arrangement of electrons in subshells (or orbitals) of an atom. It is usually expressed as a series of numbers denoting the number of electrons in each subshell (or orbital). The subshells (or orbitals) are grouped into four primary blocks: s, p, d, and f. The s block contains the s subshell, which can hold up to two electrons. The p block contains the p subshell, which can hold up to six electrons. The d block contains the d subshell, which can hold up to ten electrons. The f block contains the f subshell, which can hold up to fourteen electrons.
