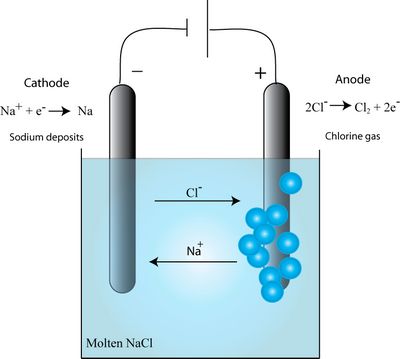
The electrolysis of molten sodium chloride involves passing an electric current through the molten salt. As a result, chlorine is released at the anode and sodium is released at the cathode. The overall reaction is as follows:
2NaCl → 2Na + Cl2

The electrolysis of molten sodium chloride involves passing an electric current through the molten salt. As a result, chlorine is released at the anode and sodium is released at the cathode. The overall reaction is as follows:
2NaCl → 2Na + Cl2