Skip to content
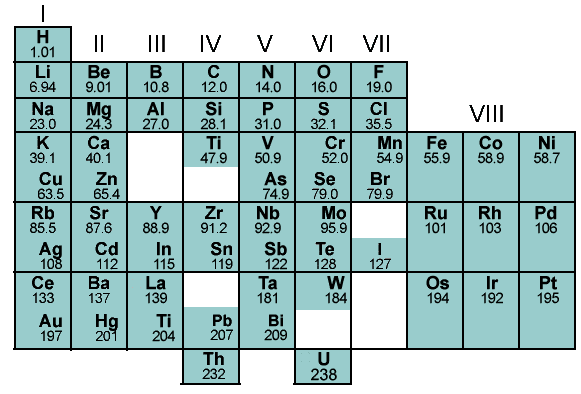
- Mendeleev’s periodic law provides an organized way to classify elements according to their atomic number and chemical properties.
- It enabled scientists to predict the existence of elements yet to be discovered and the properties they would possess.
- It provided a way to identify groups of elements with similar properties and behavior.
- It enabled scientists to develop a deeper understanding of the relationships between elements and the periodic trends of the elements.
- Mendeleev’s periodic law made it easier to predict the chemical and physical properties of elements in a group and their reaction with other elements.