CHEMISTRY
Answer any three questions from 1 to 4.(Each carries 1 score)
1).Freezing point of water is _________
2) .Name the chemical used to give red colour to food materials.
3.Which metal have high thermal conductivity?
4) .Cell used in mobile phone is________
Answer any four questions from 5 to 9.(Each carries 2 score)
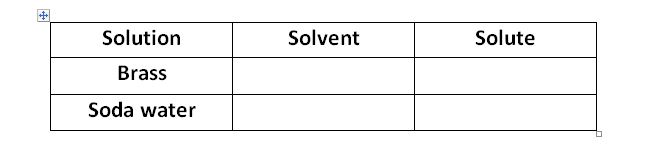
5) .Find solvent and solute from the following solution
6) .Give the reason. Sodium is always kept under kerosene.
7) . Suresh said that coconut oil is used instead of water in the radiator of motor vehicles.
a)Do you agree to his opinion? b)Justify your answer.
8) .Observe the following diagram.
a)Identify ‘A’. b)Name this reaction.
9) .You have wanted to make a supersaturated solution of common salt.How will you prepare it?
Answer any three questions from 10 to 13.[Each carries 3 score]
10) .Have you studied about Malleability,Ductility?
a)Explain these terms. b)Which metal have high ductility?
11) .Take a little dil .HCL in a test tube.Add a piece of marble(Ca2Co3)into it.
a)Which type of chemical reaction is this? b)Which gas is formed during this reaction? c)Write the chemical equation.
12) .Differentiate between colloids and suspension.
13) .Have you heard about water which can’t form lather with soap?
a)Write the name of such water? b)What is the reason for this? c)How will you remove this?
