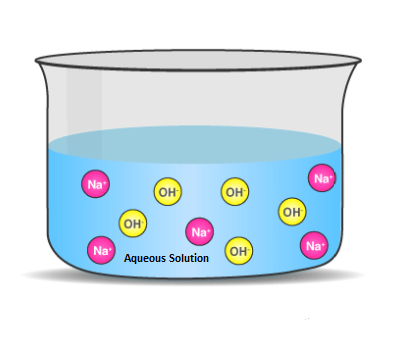
Arrhenius’ theory is a theory of acids and bases put forth by Swedish chemist Svante Arrhenius in 1887. It states that an acid is a substance that increases the concentration of hydrogen ions (H+) in a solution, while a base is a substance that increases the concentration of hydroxide ions (OH−) in a solution. This theory is still widely accepted and used to explain the behaviour of many compounds in solution.
