The basicity of acids is determined by the strength of their conjugate base. The stronger the conjugate base, the more acidic the acid. Acids with weak conjugate bases are considered weaker acids and have a lower basicity. Acids with strong conjugate bases are considered stronger acids and have a higher basicity.
Examples of acids and their basicity include:
- Hydrochloric acid (HCl): Strong acid with a high basicity
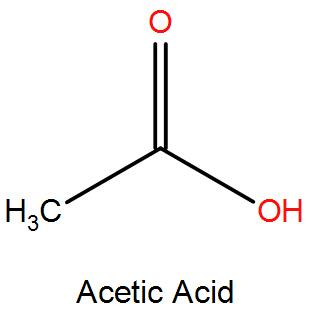
- Acetic acid (CH3COOH): Weak acid with a low basicity
- Sulphuric acid (H2SO4): Strong acid with a high basicity
- Phosphoric acid (H3PO4): Weak acid with a low basicity
