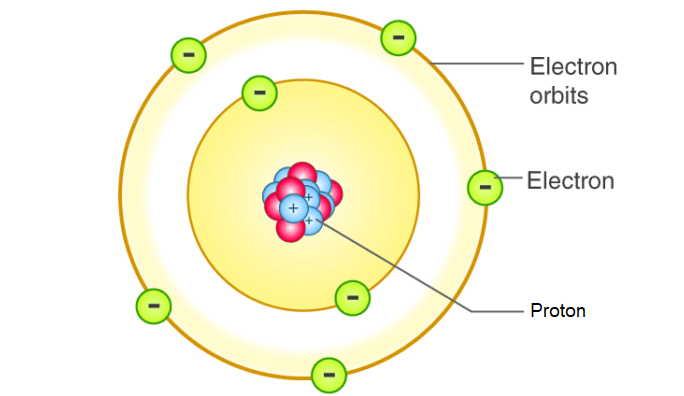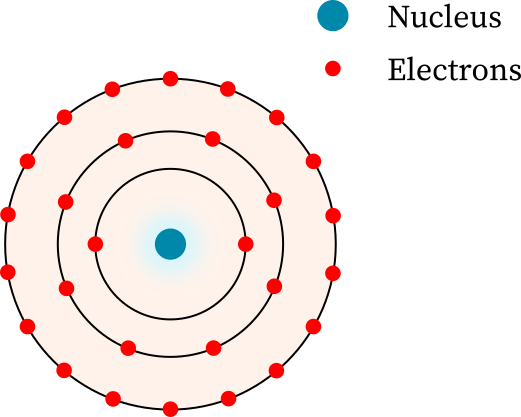
Bohr’s model of the atom is a simple representation of the structure of atoms. It was proposed in 1913 by Danish physicist Niels Bohr. The model proposed that electrons move in discrete orbits around a fixed, positively charged nucleus. The orbits are called energy levels and each has a specific energy associated with it. The energy levels are determined by the angular momentum of the electron, which is quantized in discrete units. Electrons can move from one energy level to another by absorbing or emitting electromagnetic radiation, such as visible light. 17.