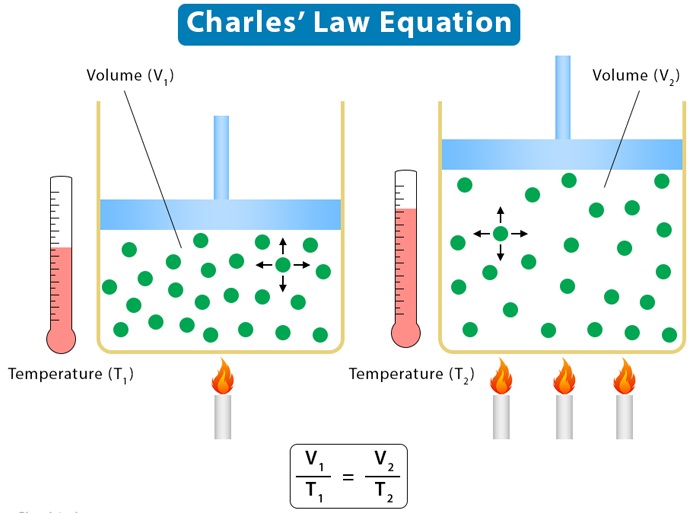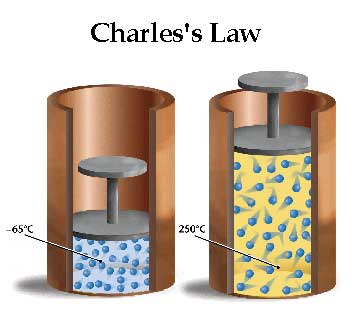
Charles’s Law, also known as the law of volumes, states that the volume of a given mass of gas is directly proportional to its temperature when pressure is held constant. This law was discovered by French physicist Jacques Charles in 1787 and is stated mathematically as V/T=k, where V is the volume, T is the temperature (in Kelvin), and k is a constant. Charles’s law is one of the four gas laws that together form the ideal gas law.