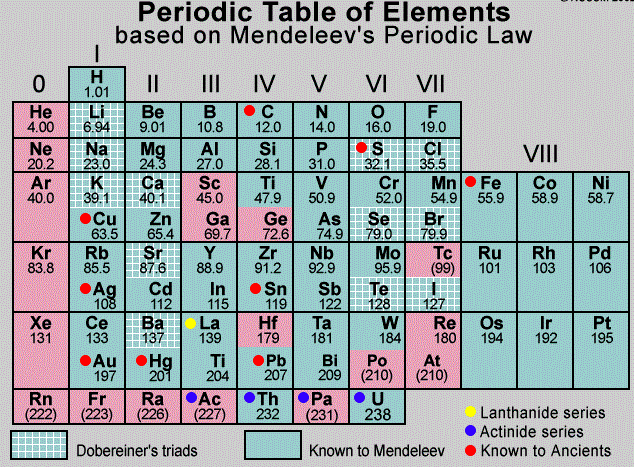
The Mendeleev classification is a system of chemical elements developed by Russian scientist Dmitri Mendeleev in 1869. It arranges the elements in order of increasing atomic number and groups them into eight categories based on their similar properties. These categories are labeled as groups and periods. Each group contains elements with similar chemical properties while each period contains elements with similar atomic structures. The table below shows the Mendeleev classification for the elements in the periodic table.
Group | Period | Element
1 | 1 | Hydrogen
2 | 2 | Helium
3 | 3 | Lithium
3 | 4 | Beryllium
4 | 5 | Boron
5 | 6 | Carbon
6 | 7 | Nitrogen
7 | 8 | Oxygen
8 | 8 | Neon
1 | 2 | Sodium
2 | 3 | Magnesium
3 | 4 | Aluminium
4 | 5 | Silicon
5 | 6 | Phosphorus
6 | 7 | Sulphur
7 | 8 | Chlorine
8 | 8 | Argon
Mendeleev
