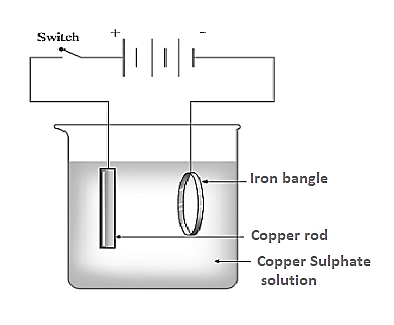
Copper plating on iron bangle is a process that involves electroplating a thin layer of copper onto the surface of an iron bangle. This is done by submerging the iron bangle in a copper sulphate bath that has been electrically charged. An electrical current is then passed through the copper sulphate solution, causing copper ions to be attracted to the iron bangle. The copper ions attach to the iron and form a thin layer of copper on its surface. This process not only gives the bangle a unique look, but also helps to protect it against corrosion, as copper is a very durable and corrosion-resistant metal.
Cu(s) + Fe(NO3)2(aq) –> Cu(NO3)2(aq) + Fe(s)
The reaction is an oxidation-reduction reaction in which copper is reduced and iron is oxidized. Copper is deposited onto the iron bangle in the form of Cu2+ ions that are reduced to metallic copper.
