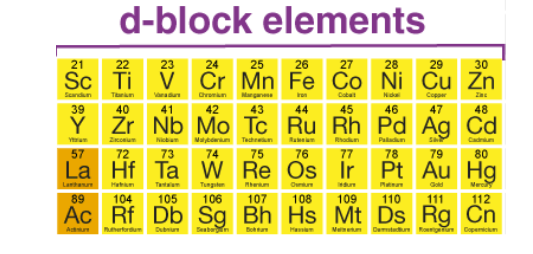
The d-block elements consist of the elements in the middle three columns of the periodic table. These elements are the transition metals. The d-block elements are characterized by having their outermost valence electrons in a d-orbital. The d-block elements are generally hard, have higher melting points, and are more resistant to chemical reactions. Examples of d-block elements include iron (Fe), copper (Cu), cobalt (Co), and nickel (Ni).
