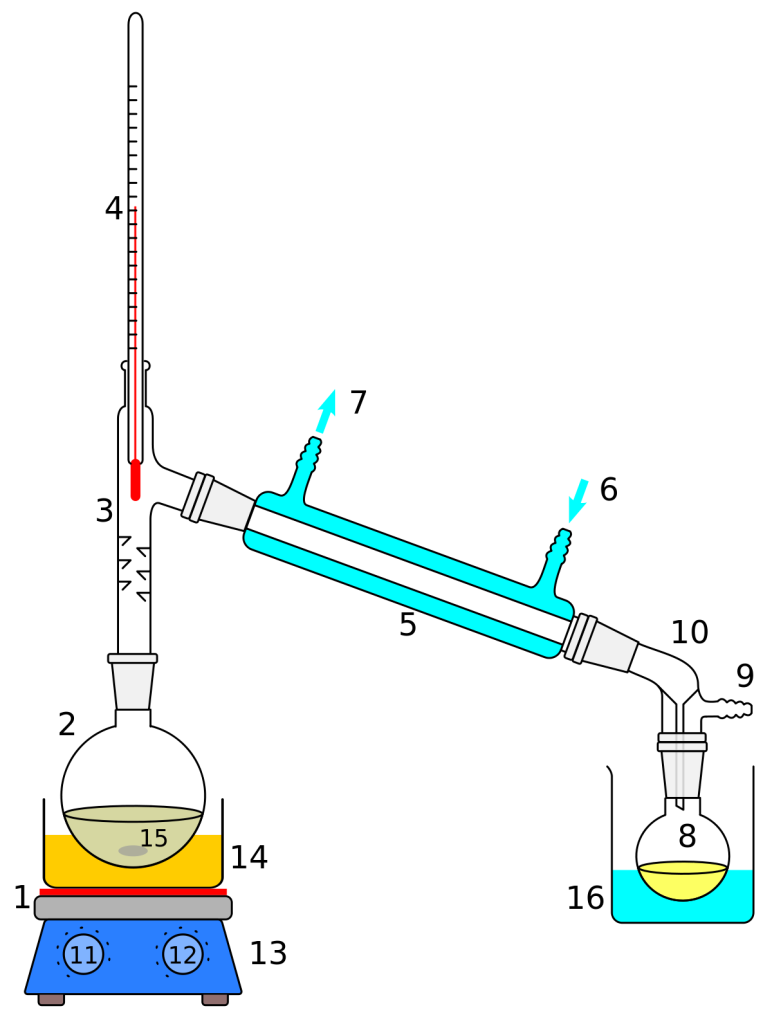
Distillation is a process used to separate a liquid mixture into its component parts, or fractions. In the case of a salt solution, it is possible to separate the water from the salt by distillation.
To perform a distillation, a round bottom flask is filled with the salt solution and attached to a condenser. The condenser is connected to a cold water source. Heat is applied to the flask, typically with a Bunsen burner, and the solution begins to boil. As the liquid begins to boil, the vapor rises and is cooled in the condenser, turning back into a liquid. This liquid, which is mostly water with much less salt, is collected in a receiving flask.
Observation: As the liquid in the round bottom flask begins to boil, the vapor is seen rising and condensing in the condenser. The condensate, which is mostly water with a much lower concentration of salt, is collected in a receiving flask.

