The Dobereiner periodic table was created by German chemist Johann Wolfgang Dobereiner in 1829. It was the first attempt to organize the elements into a periodic table. The table was based on the idea of triads, which are groups of three elements with similar properties. He noticed that the mass of the middle element of each triad was roughly the average of the masses of the other two elements. For example, the elements chlorine, bromine, and iodine form a triad, with the middle element being bromine whose mass is roughly the average of the other two elements.
The limitation of triads is that they are limited to three notes. While there are many different ways to arrange and play with three notes, the musician is ultimately restricted in the amount of harmonic material they can create with the combination of the three notes. Additionally, because the notes of a triad are all related to each other, the musician is limited to the same type of harmonic relationship between the notes.
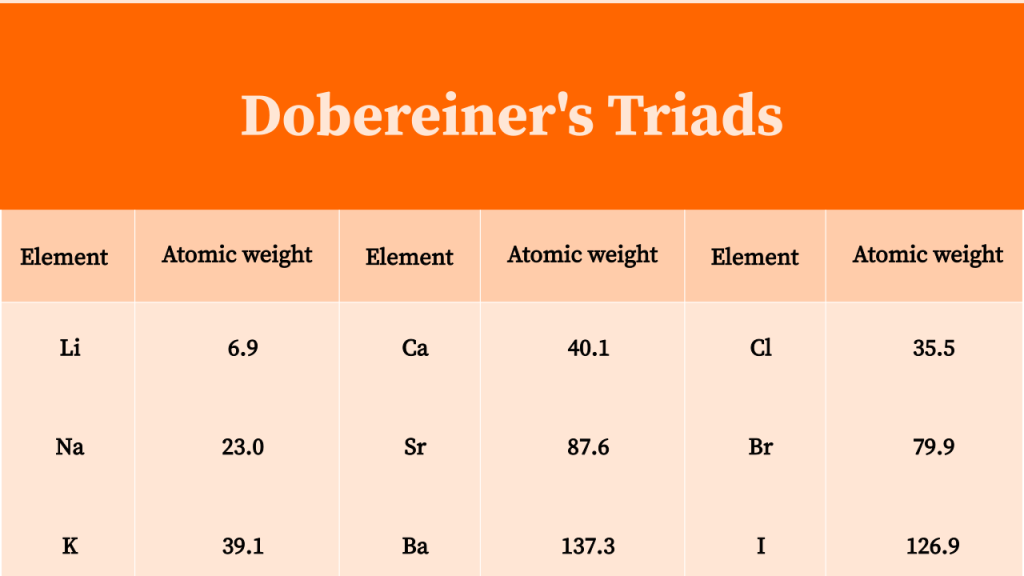
periodic table, triads
