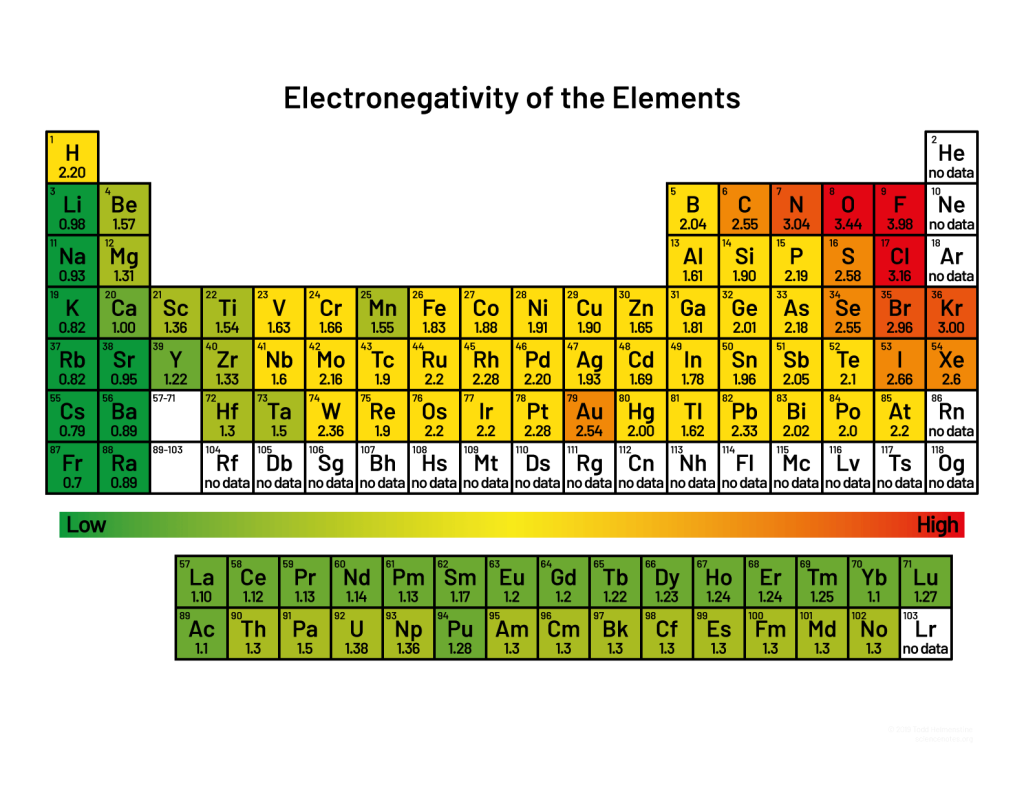
Electro negativity is a measure of the ability of an atom or molecule to attract electrons when forming a chemical bond. It is measured on a scale from 0.7 to 4.0, with higher numbers indicating greater electro negativity. Fluorine is the most electronegative element, with a value of 4.0, while cesium has the lowest electro negativity, at 0.7.
Examples of electro negativity include the following:
• Oxygen has an electro negativity of 3.5, making it highly electronegative.
• Carbon has an electro negativity of 2.5, making it moderately electronegative.
• Sodium has an electro negativity of 0.9, making it slightly electronegative.
• Fluorine has an electro negativity of 4.0, making it the most electronegative element.
