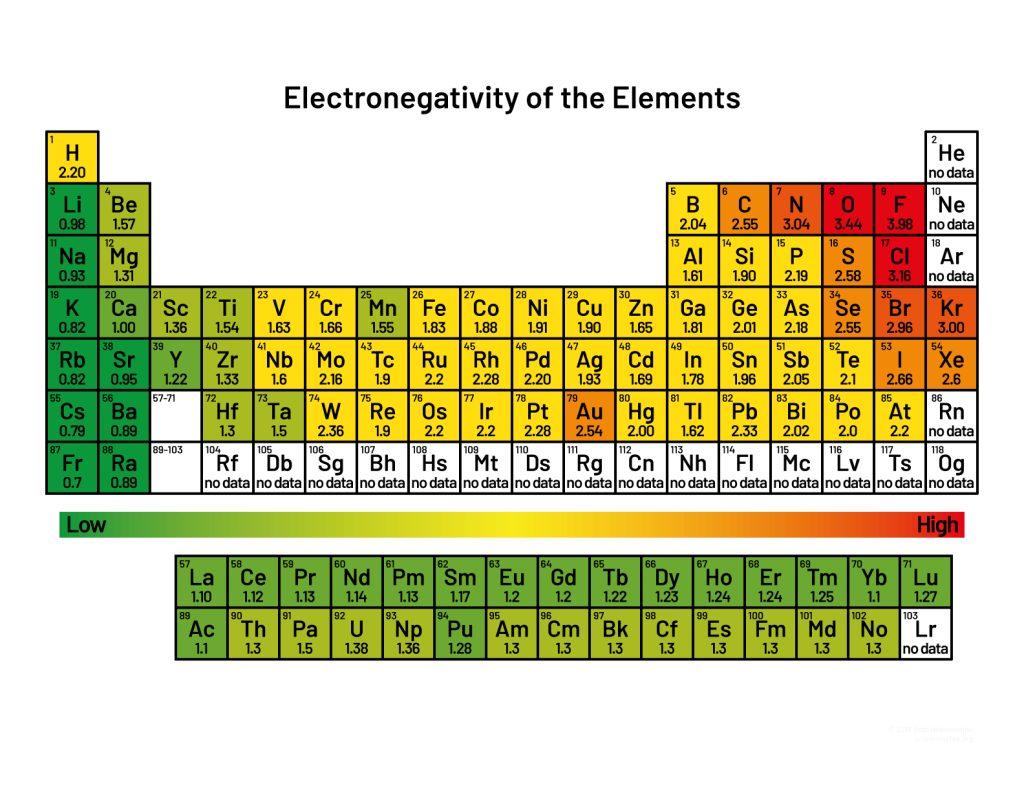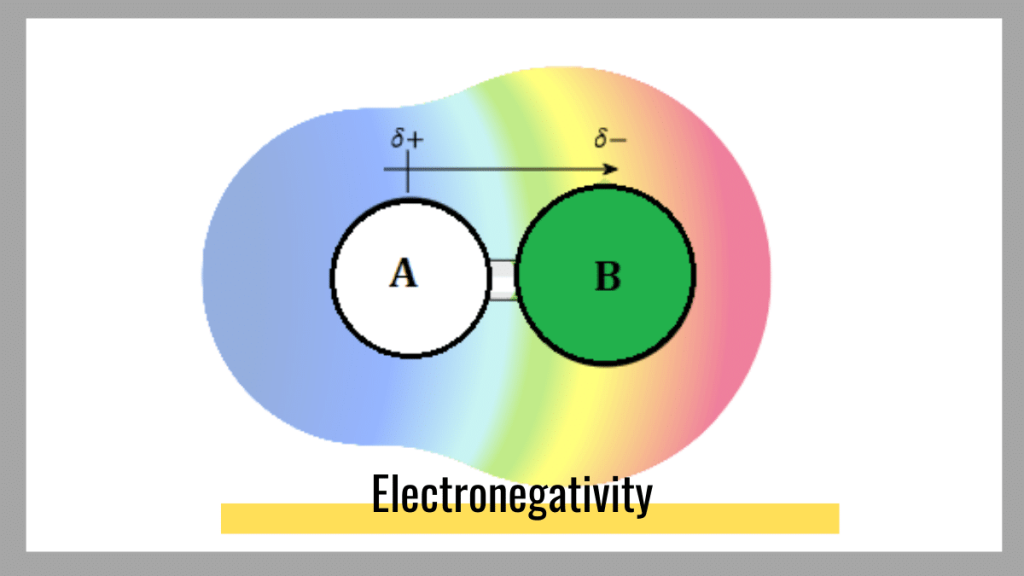
Electro negativity is a measure of the tendency of an atom to attract electrons towards itself. It is measured on an electro negativity scale, which ranges from 0 (for a very weakly electronegative atom) to 4 (for a very strongly electronegative atom).
Examples of atoms with high electro negativity include fluorine (3.98), oxygen (3.44), and chlorine (3.16). Examples of atoms with low electro negativity include sodium (0.93), potassium (0.82), and magnesium (1.31).