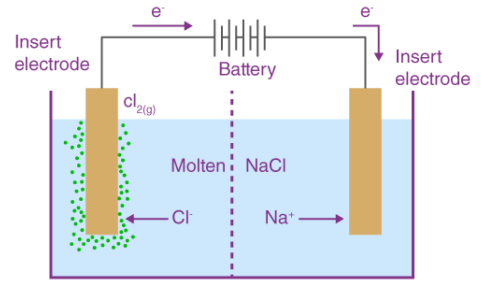
Electrolytic cells are electrochemical cells that use electricity to drive a non-spontaneous reaction. This electricity is typically provided by an external source, such as a battery or power supply. Inside the cell, the electrical current causes ions to move between two electrodes, resulting in a chemical reaction that produces a product. This product can be a gas, liquid, or solid, depending on the reaction taking place. Electrolytic cells are widely used in industrial processes, such as the production of chlorine and other chemicals, electroplating, and electro refining.
An electrolysis cell oxidation experiment is an experiment that uses electricity to cause a chemical reaction. This type of experiment is often used to show the oxidation process of metals, such as iron or copper. The experiment requires the setup of an electrolysis cell, which consists of two electrodes that are connected to a power supply. The electrodes are placed in a solution of electrolyte, which is typically a salt, such as sodium chloride. The power supply causes the electrolyte to become ionized, and the oxidation process begins. When the metal is oxidized, the electrons from the metal are transferred to the electrolyte, resulting in the formation of an oxide. The experiment can be used to show the reaction between different metals when exposed to different concentrations of electrolyte, as well as the effect of temperature and current on the oxidation process.
