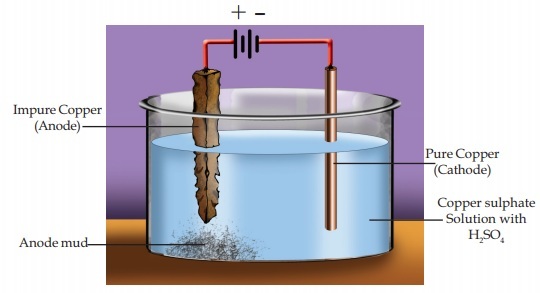
Electrolytic refining is a process that is used to purify the metal and remove any impurities that may be present. The electrolytic refining process is a technique that is used to refine various kinds of metals such as copper, gold, silver, nickel, and lead.
The process starts with the metal being cast into an anode, which is a form of the metal that has been purified. This anode is then placed in an electrolytic bath filled with a solution of acid and metal salts. An electric current is then passed through the metal, which causes the metal to break down into its component ions and release the impurities. The impurities are then removed from the solution and the metal is left in its pure form.
The electrolytic refining process is used to produce high purity metal products and to help ensure that the metal is free from any impurities. It is a very efficient process and is often used in the production of precious metals
