The electronic configuration of elements in the periodic table is based on the number of electrons in the atom. The configuration is determined by the number of protons in the nucleus and the arrangement of electrons in shells.
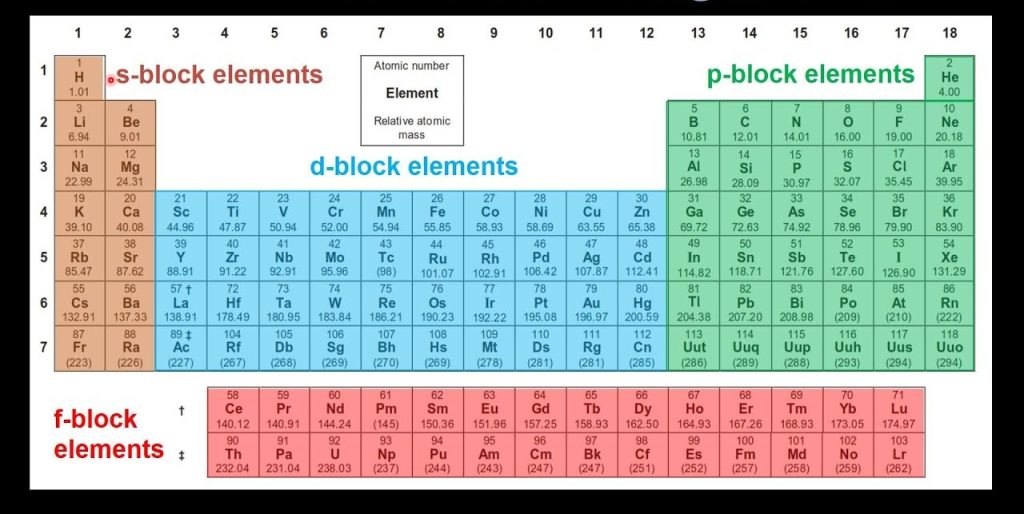
The elements in the periodic table are arranged in order of increasing atomic number, which is the number of protons in the nucleus. Each element has its own unique atomic number, which is represented by a number in the upper right corner of the element’s box.
The elements are arranged in rows and columns, with each row representing a period and each column representing a group. The periods are numbered 1 to 7, while the groups are labeled with letters from A to G.
The elements in the same group have similar chemical properties and electron configurations, while the elements in the same period have similar atomic radii. As you move down a group, the atomic radius increases and the number of shells increases. As you move across a period, the atomic radius decreases and the number of shells remains the same.
The elements in the periodic table can also be divided into metals, non metals, and metalloids. Metals are found on the left side of the table, non metals on the right side, and metalloids in the middle.
The periodic table is divided into 18 groups and 7 periods. The groups are numbered from 1 to 18, and the families within each group include:
- Group 1: Alkali Metals
- Group 2: Alkaline Earth Metals
- Group 3: Boron Group
- Group 4: Carbon Group
- Group 5: Nitrogen Group
- Group 6: Oxygen Group
- Group 7: Halogens
- Group 8: Noble Gases
- Group 9: Rare Earth Metals
- Group 10: Transition Metals
- Group 11: Transition Metals
- Group 12: Transition Metals
- Group 13: Boron Group
- Group 14: Carbon Group
- Group 15: Pnictogens
- Group 16: Chalcogens
- Group 17: Halogens
- Group 18: Noble Gases
