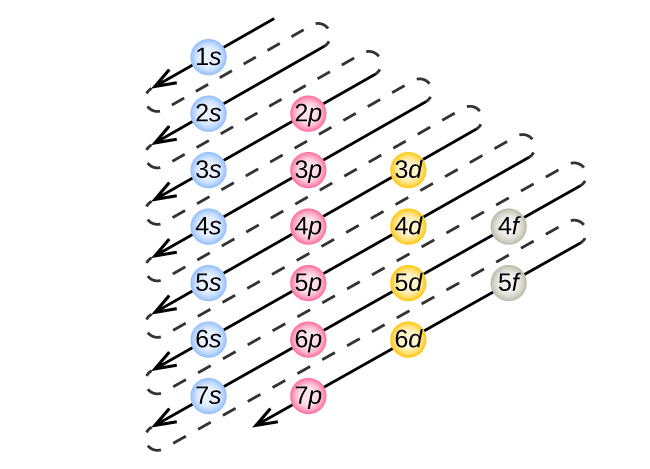
The electronic configuration of an atom is the arrangement of electrons in its various atomic orbitals. An atomic orbital is the region around an atom where electrons are most likely to be found. The electronic configuration of an atom is determined by the number of protons and neutrons in the nucleus, as well as the energy levels of the electrons.
Examples:
- Hydrogen: 1s1
- Oxygen: 1s2 2s2 2p4
- Carbon: 1s2 2s2 2p2
- Sodium: 1s2 2s2 2p6 3s. Gold: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s1
