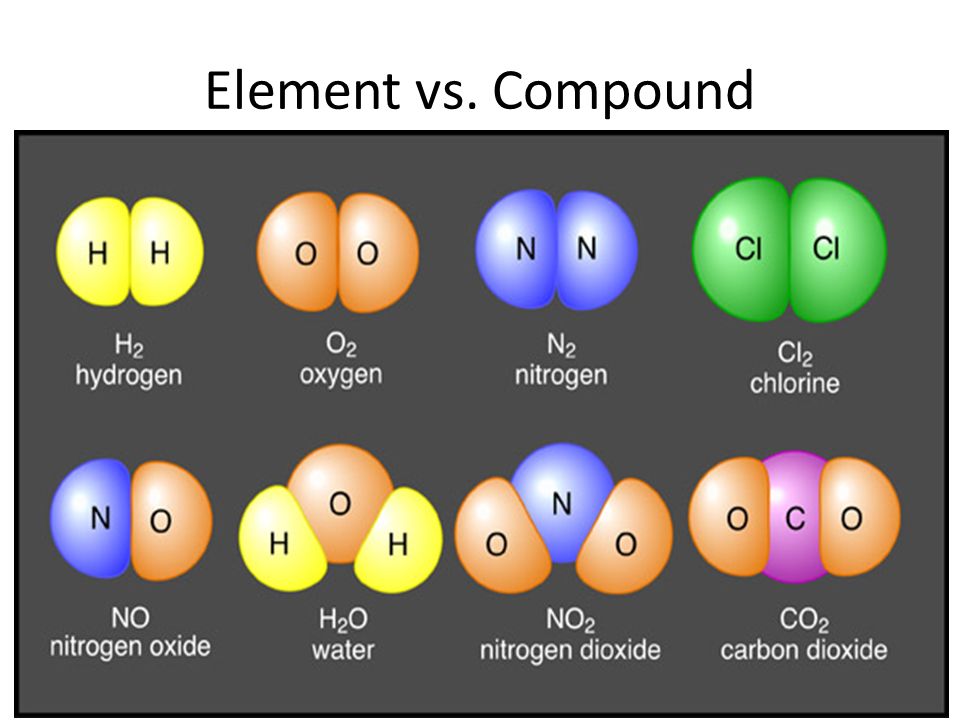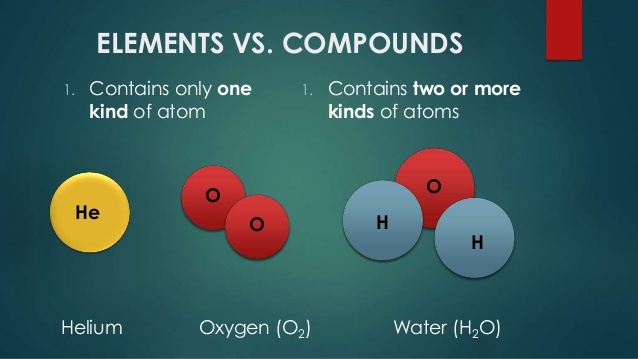
In chemistry, elements are the simplest forms of matter, consisting of one type of atom. There are 118 elements found in nature, divided into metals, nonmetals, and metalloids. Examples of elements include hydrogen, oxygen, carbon, and iron. The pure substance which cannot be further decomposed through chemical process is called elements.
Compounds in chemistry are molecules or ions that are composed of two or more different elements chemically combined in a fixed ratio. Examples of compounds include water (H2O), table salt (NaCl), and carbon dioxide (CO2). Compounds are the pure substances formed from two or more elements through chemical combination.
Elements and compounds are both pure substances. Elements are made up of only one type of atom, while compounds are made up of two or more different types of atoms bonded together. Elements cannot be broken down into simpler substances, while compounds can be broken down into elements or simpler compounds.