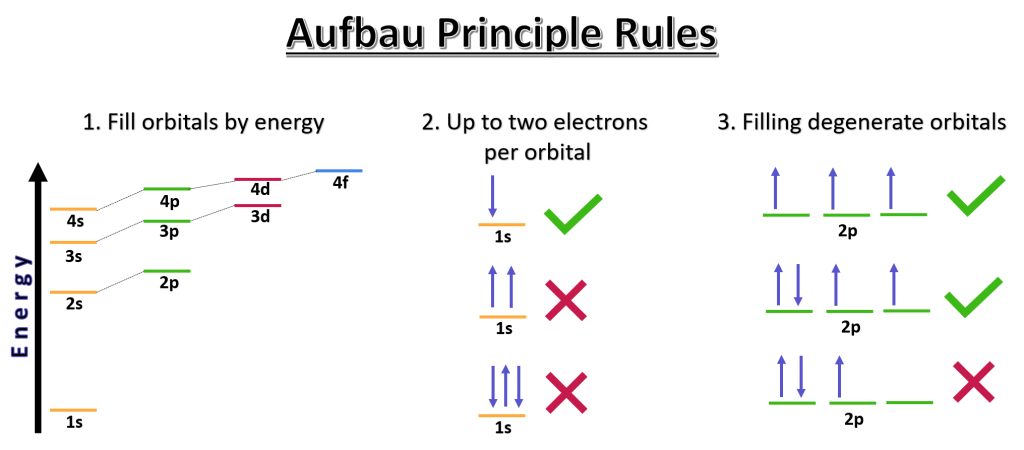
The electrons in an atom fill up the orbitals in the increasing order of energy levels. This is known as the Aufbau principle. The orbitals are filled up in the order of s, p, d and f, starting from the lowest energy level. The maximum number of electrons that can fill up an orbital is two, and each electron has an opposite spin. This is known as the Pauli exclusion principle.
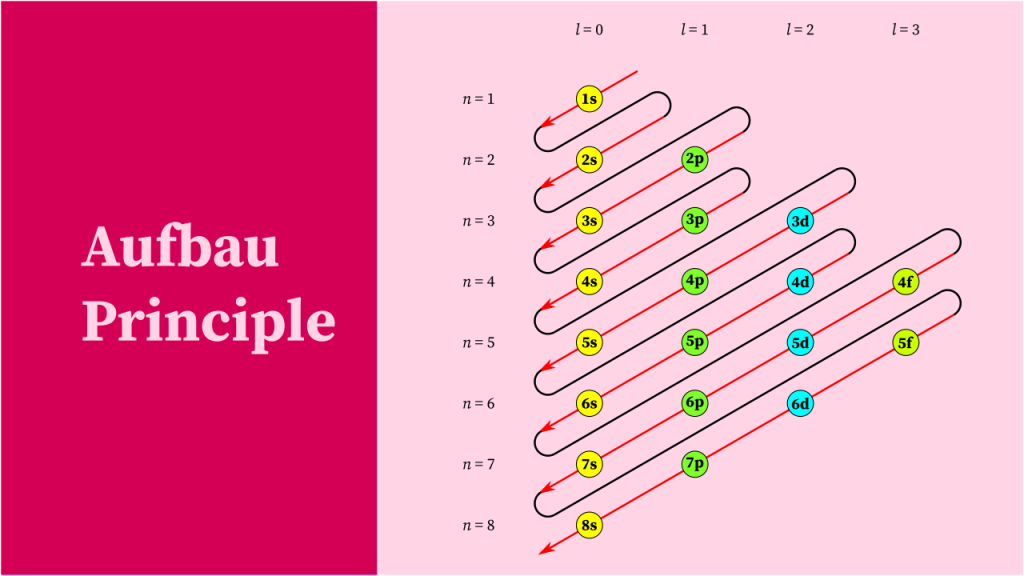
- Electron configuration is the arrangement of electrons in an atom or molecule.
- Electron configuration is written using the element’s symbol, followed by superscript numbers indicating the number of electrons in each energy level.
- The outermost energy level is written first, followed by each successive energy level.
- Electron configurations are written in order of increasing energy level, with each energy level containing a maximum of two electrons.
- The total number of electrons in an atom or molecule is equal to the atomic number of the element.
- Electron configuration can be used to determine the element’s chemical properties and reactivity.
