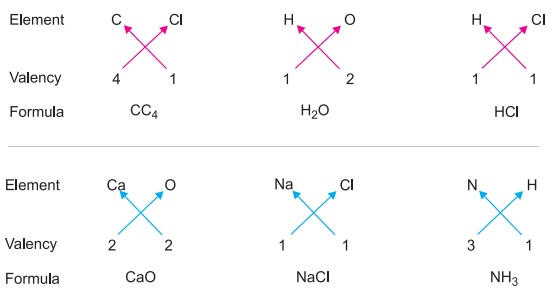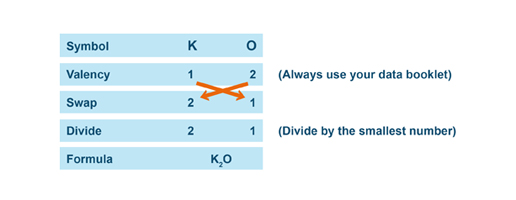
Valency is the number of chemical bonds that an atom can form with other atoms. This information can be used to determine the chemical formula of a compound, which is the ratio of the elements in the compound. For example, if an atom has a valency of 2, it typically forms a compound with another atom that has a valency of 2, creating a formula of 1:1, or A:B. If the atom has a valency of 3, it typically forms a compound with another atom that has a valency of 5, creating a formula of 3:5, or A3B5.