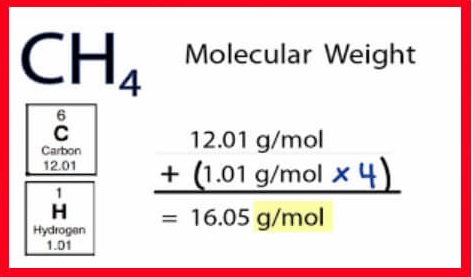
The gram atomic mass is the mass in grams of one mole of atoms of a particular element. It is equal to the atomic weight in atomic mass units multiplied by Avogadro’s number (6.02 x 10^23).
Gram atomic mass = (Number of moles of atoms) × (Atomic mass of element) 12.
