Skip to content
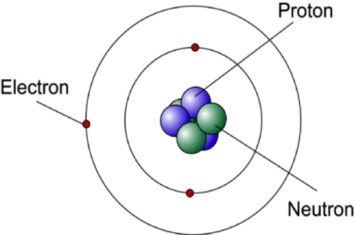
- Electrons orbit the nucleus of an atom in specific, discrete energy levels.
- When electrons jump from a higher energy level to a lower energy level, they release energy in the form of photons.
- Electrons can only exist in certain, discrete energy levels and cannot have any energy in between.
- The electrons are held in their orbits by electrostatic attraction between the positively charged nucleus and the negatively charged electrons.
- Electrons are both particles and waves, exhibiting both wave-like and particle-like behaviour.