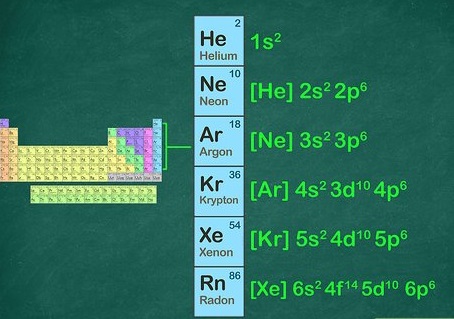
Noble gas electronic configuration refers to the complete electron configuration of the outermost shell of a noble gas, which is a group of elements in the periodic table that are highly stable due to having full valence shells. The noble gas electronic configuration for the first six noble gases is as follows:
- Helium (He): 1s2
- Neon (Ne): 1s2 2s2 2p6
- Argon (Ar): 1s2 2s2 2p6 3s2 3p6
- Krypton (Kr): 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6
- Xenon (Xe): 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
- Radon (Rn): 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6
