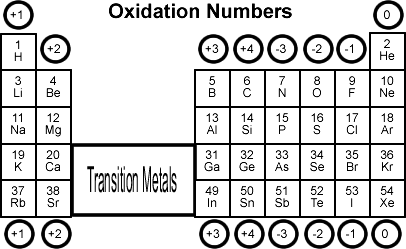
The oxidation number of an atom in a compound can be determined by using a set of rules, such as the following:
- The oxidation number of an atom in a neutral element (e.g., H2, O2, Cl2) is zero.
- The oxidation number of a monatomic ion is equal to its charge.
- The oxidation number of a Group IA element in a compound is +1.
- The oxidation number of a Group IIA element in a compound is +2.
- The oxidation number of a Group VIIA element in a compound is -1.
- The oxidation number of a Group VIIIA element in a compound is -2.
- The oxidation number of hydrogen in a compound is usually +1, except when it is bonded to a metal, in which case it is -1.
- The oxidation number of oxygen in a compound is usually -2, except in peroxides and compounds containing fluorine, in which case it is -1.
- The sum of the oxidation numbers of all atoms in a compound is equal to the charge on the compound.
