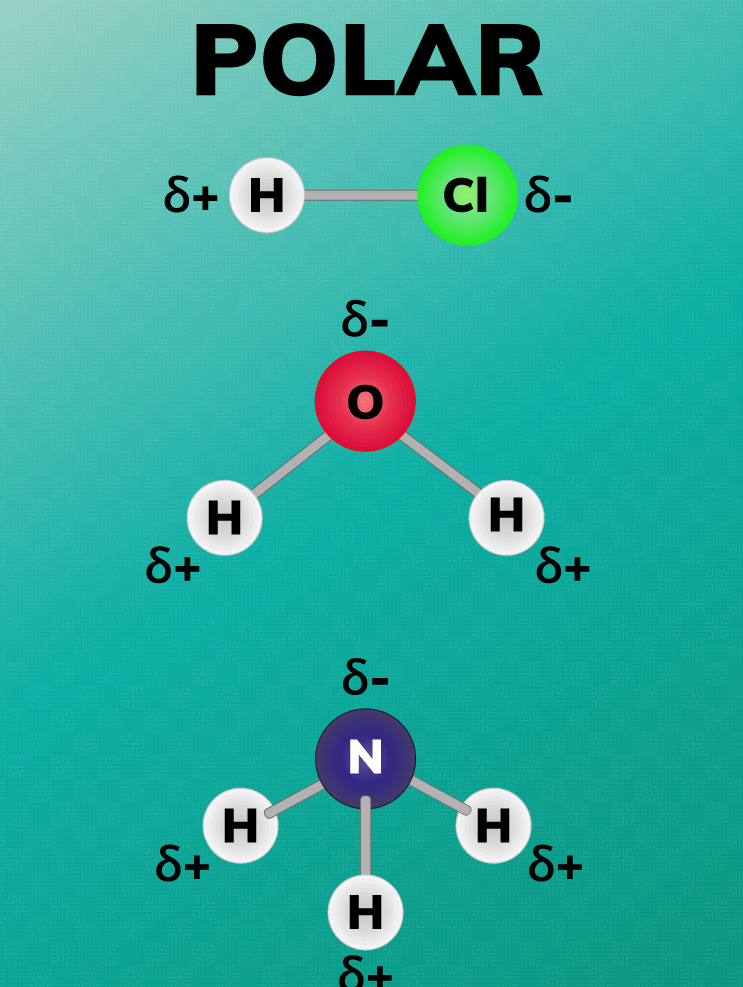
Polar compounds are molecules that have an uneven distribution of electrons, making one end of the molecule more positive than the other. This creates a dipole moment, where the positive end of the molecule is attracted to the negative end of another molecule. Examples of polar compounds include water, ethanol, acetic acid, and ammonia.
