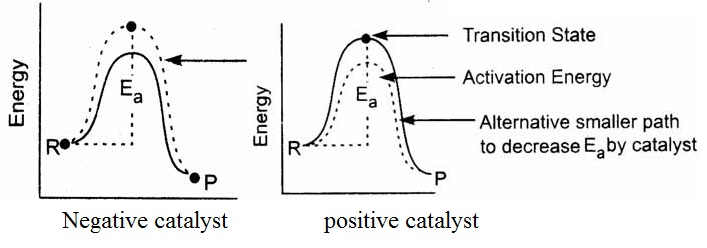
Positive catalyst: A positive catalyst is a substance that increases the rate of a chemical reaction without itself being consumed. These catalysts are usually enzymes or chemicals that help to speed up a reaction by providing an alternative reaction pathway with a lower activation energy.
Negative catalyst: A negative catalyst is a substance that decreases the rate of a chemical reaction without itself being consumed. These catalysts can be either inhibitors or poisons that slow down a reaction by causing a reaction pathway with a higher activation energy. example, cobalt chloride is a negative catalyst for the oxidation of hydrogen peroxide.
