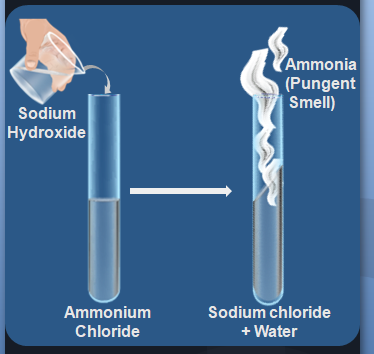
In the laboratory, ammonia can be prepared by combining an aqueous solution of ammonium chloride and sodium hydroxide. The reaction is as follows:
NH4Cl + NaOH → NaCl + H2O + NH3
The resulting ammonia solution can then be collected in a gas-tight container such as a bottle or flask.
To prepare the solution, an aqueous solution of ammonium chloride is first prepared by dissolving the solid in water. This solution is then added to a solution of sodium hydroxide in a beaker or flask. The reaction is usually exothermic (heat is released) and vigorous stirring is required to ensure that the reaction goes to completion.
Once the reaction is complete, the ammonia gas is collected in a gas-tight container such as a bottle or flask. The container should be cooled with ice during the collection process to ensure that the ammonia gas is not lost. The ammonia solution can then be used in various laboratory experiments or applications.
