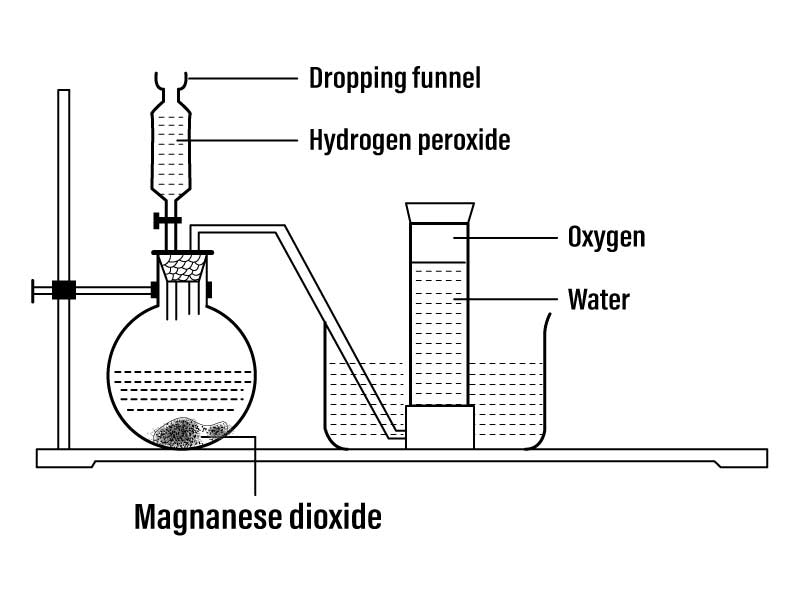
Potassium permanganate (KMnO4) is an inorganic compound used to prepare oxygen gas in a laboratory setting. The reaction involves the oxidation of manganese (IV) oxide in an acidic solution, resulting in the release of oxygen gas. The reaction is as follows: 2KMnO4 + 3H2SO4 → K2SO4 + 2MnSO4 + 3H2O + 3O2. The reaction is exothermic, meaning it releases heat, and can be used to produce a hot flame. The end product is a mixture of oxygen gas and some un reacted potassium permanganate.
