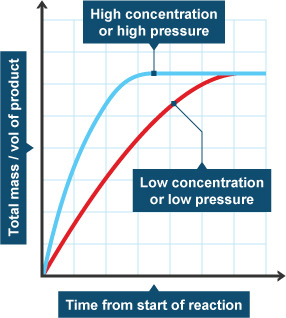
Concentration is one of the main factors that affect the rate of a chemical reaction. As the concentration of reactants increases, the reaction rate increases as well. This is because when the concentration of reactants is increased, there are more molecules of reactants available to react, meaning more collisions can occur and the reaction rate increases.
