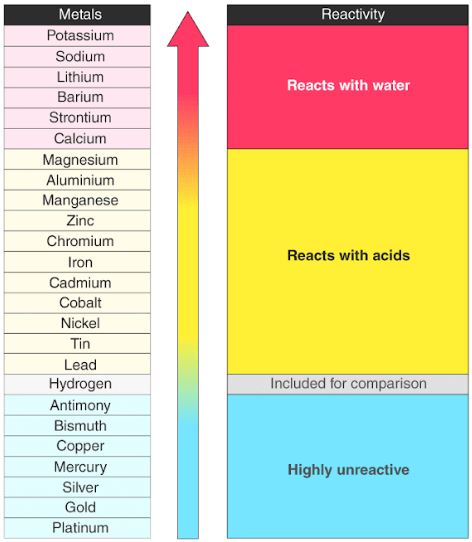
Reactivity series:
The reactivity series is a list of metals, organized from most reactive to least reactive. The most reactive metals are at the top of the list and include potassium, sodium, calcium, magnesium, aluminium, zinc, iron, and lead.
Displacement reactions:
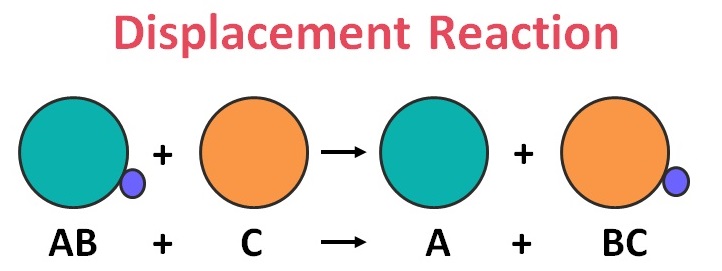
A displacement reaction is a type of chemical reaction where one element is displaced by another, usually more reactive, element. This is usually a redox reaction where the more reactive element is oxidized and the less reactive element is reduced. The reaction can be represented by the equation A + BC → B + AC, where A is the less reactive element and B is the more reactive element.
When a zinc rod is dipped in a copper sulphate solution, the zinc metal will react with the copper sulphate to form zinc sulphate and copper metal. The equation for this reaction is:
Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
The observation of this reaction is that the zinc rod will begin to dissolve in the solution and turn a blue colour, due to the formation of zinc sulphate in the solution. Additionally, a copper metal deposit will form on the zinc rod, due to the reduction of copper ions from the solution.
Zinc and copper sulphate solution:
Zinc + Copper Sulphate -> Zinc Sulphate + Copper
Iron and copper sulphate solution:
Iron + Copper Sulphate -> Iron Sulphate + Copper
