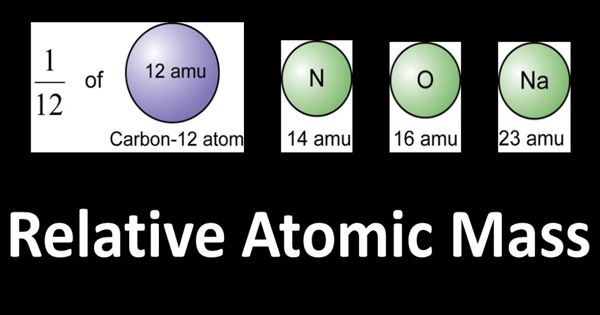
The relative atomic mass (abbreviated as Ar) is the ratio of the average mass of atoms of an element to 1/12 of the mass of a carbon-12 atom. It is an average atomic mass of an element, calculated from the weighted average of all isotopes of an element, taking into account the abundance of each isotope.
